Arm yourself with a basic understanding of acids and bases. It is a little confusing at first, but learning the role of pH is important for the effective removal of dirt.
Different pH values will clean different types of dirt. Understanding the material that you are cleaning and the type of dirt will help you select the correct cleaning product. Giving you the results you want without damaging the material.
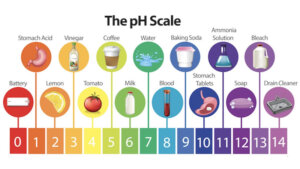
It is essential to understand that everything has a pH value. You can find out how acidic an object is by using the pH scale. We number a scale 1 to 14. The values 0 to 6 are acidic, 7 neutral, and 8 to 14 alkaline.
The difference between pH values when dealing with the pH scale is that they are logarithmic and not linear. Each value is increased tenfold as you move it down the scale. for example:
Do not be fooled by the proximity and numbers on the scale. The difference between something sitting at pH 11 and pH 13 is huge. Not understanding how pH works can damage what you are cleaning, and may cause major harm to yourself and others.
They classify cleaners as either neutral, acidic, or alkaline. The higher the pH level of a solvent, the more corrosive it is.
These cleaners have a pH between 6 and 8 and are used for general daily cleaning of surfaces. We also use them for cleaning more delicate materials such as wool, silk, and cotton.
With the added advantage of causing less harm if curious kids or animals get their hands or paws on them.
Neutral cleaners are milder, making them less toxic and dangerous. However, they are also less effective.
Acid cleaners have a pH below 6, and we use them for cleaning inorganic mineral-based dirt. One of the main areas that we use it in is the bathroom.
Cleaners with a low pH can be used to clean toilet bowls, remove lime scale or soap scum, and are useful for degreasing.
In carpet, upholstery, and fabric cleaning, they can be useful for removing tannic (tea, coffee, and wine) and rust stains. Also, because of their iron content, they can be useful for removing blood stains.
This category of cleaners has a pH of 8 and above. This falls into the most common category of available cleaning products.
General-purpose cleaners have a pH between 9 and 11. They can be used to remove oils, particulate soiling, fats, sugars, organic soils, and proteins.
Laundry detergents, baking soda, and a variety of other cleaning supplies that you may get at the grocery are some examples of these.
Heavy-duty cleaners and degreasers usually have a pH of 13 to 14. They can be used to remove heavy grease, unblock drains, clean ovens, remove oil, and for other tasks that require a high level of causticity. Caustic sodium, soda, and ammonia are good examples of high-pH degreasers and cleaners.
These high-pH products are the most likely to cause damage to the item you are cleaning and toxic fumes being released. As well as causing injury to the user if protection is not used.
Different pH levels will remove different forms of soil.
We have encountered numerous cleaning assignments where the items have been irreparably damaged. This could have been avoided in the first place if the proper pH-cleaning product had been used.
We have also made our cleaning products that are not only eco-friendly but also insanely cheap. They are so effective, that we have thrown out our commercial cleaners and replaced them with these.